
by Alexander Perez | Nov 10, 2021 | Uncategorized
Tepnel is a GMP quality assured analytical testing facility, with over 30 years of expertise and knowledge in a wide range of cost-effective services for the whole drug development process. Our team were recently approached to develop two new methods for peptide drug... Read more 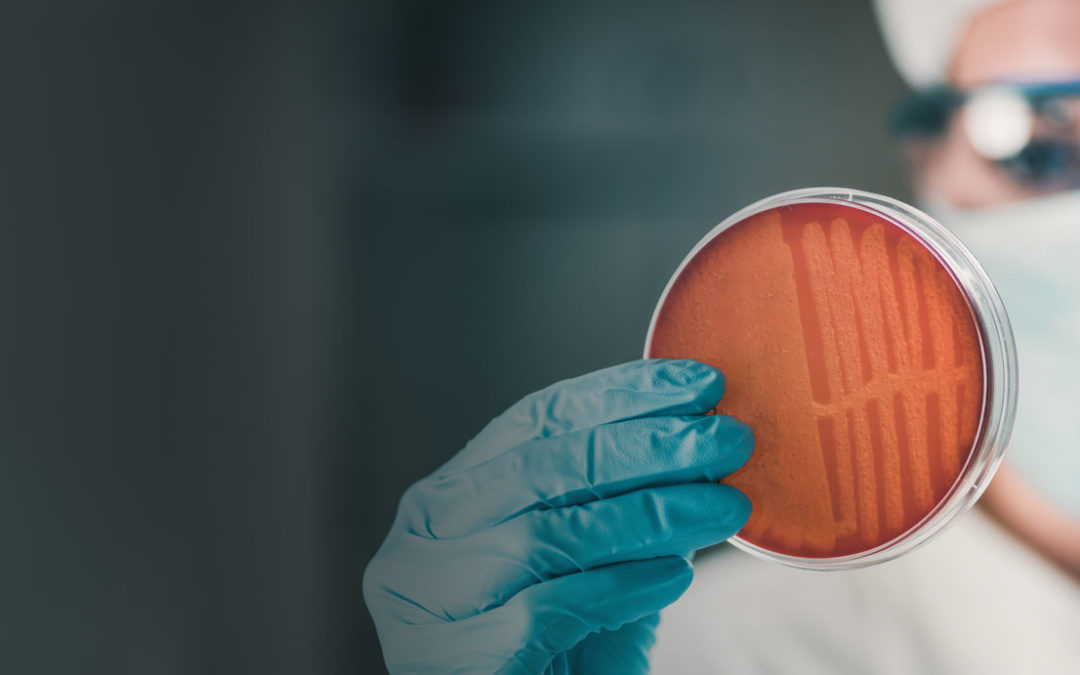
by Alexander Perez | May 27, 2021 | Uncategorized
Microbial contamination can present a number of problems when it comes to pharmaceutical preparations and their ingredients. Which is why it’s imperative for patient safety, that microbial specifications and testing protocols are developed for the whole manufacturing... Read more 
by Alexander Perez | Feb 3, 2021 | Uncategorized
Get in touch +44 (0)1506 424270 Send us a message FollowFollowFollow Is the clock now ticking for European Pharmaceutical manufacturers to select their Lot and Batch Release testing laboratory in the UK to transfer their required methods before the... Read more 
by Alexander Perez | Dec 10, 2020 | Uncategorized
At Tepnel, we have over 40 years of experience in the pharmaceutical field. Focussing on analytical testing such as lot and batch release testing, raw materials testing, method development and stability testing and storage. We have and always will strive to provide... Read more 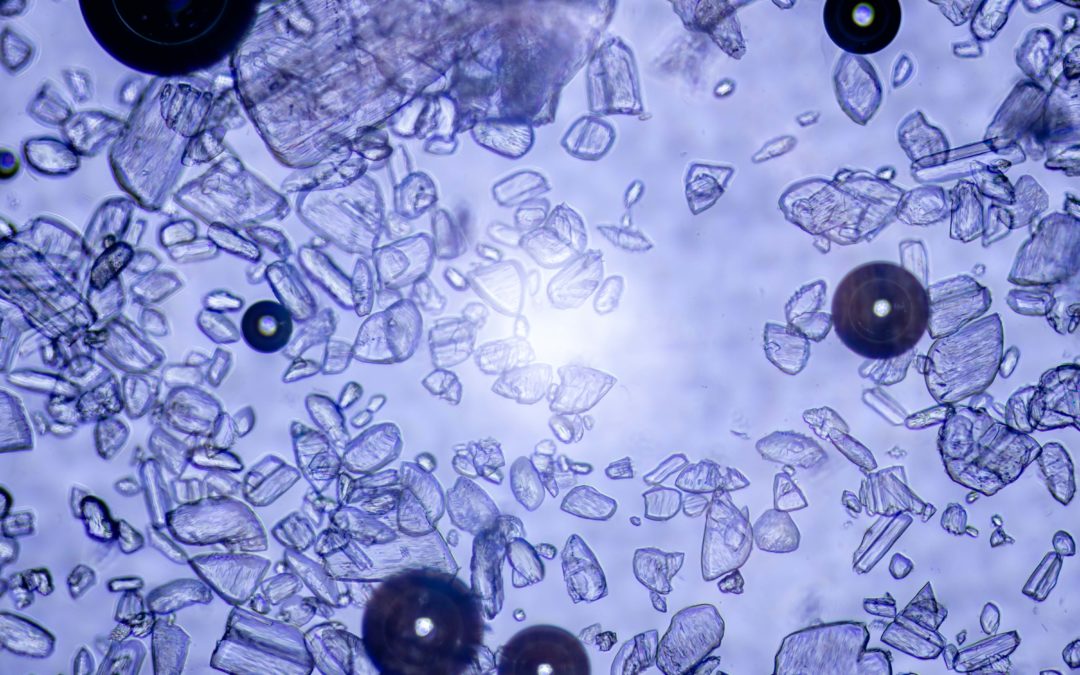
by Alexander Perez | Nov 10, 2020 | Uncategorized
Tepnel is excited to announce that the small volume sub-visible particle adaptor for our HIAC instrument is now installed and qualified, ready to enhance our analytical testing to further meet your needs. To find out more about how we can support your drug development... Read more




